Clinical Studies on Generic Drug Efficacy: What the Research Really Shows
 Jan, 23 2026
Jan, 23 2026
When you pick up a prescription, you might see two names on the bottle: one you recognize from TV ads, and another that’s cheaper-often much cheaper. That’s the generic version. But does it work the same? People worry. Doctors wonder. Pharmacists explain. And behind all of it, there’s a mountain of real-world data from clinical studies spanning decades.
What does ‘generic’ actually mean?
A generic drug isn’t a copy. It’s not a knockoff. It’s required by law to contain the exact same active ingredient, in the same strength, and delivered the same way-as the brand-name drug. That means if you’re taking metformin for diabetes, whether it’s branded Glucophage or a generic version, the molecule doing the work is identical. The FDA, EMA, and other global regulators demand this. The inactive ingredients-fillers, dyes, coatings-can differ. But those don’t affect how the drug works in your body. They just change how it looks or tastes.How do we know generics work the same?
Before a generic drug hits the shelf, it must pass a test called bioequivalence. This isn’t theoretical. It’s done in real people. Typically, 24 to 36 healthy volunteers take both the brand and generic versions in a crossover study-meaning half take the brand first, then the generic; the other half do the reverse. Researchers measure how much of the drug enters the bloodstream and how fast. The standard? The generic’s absorption must fall within 80% to 125% of the brand’s. That’s not a wide gap. It’s tight. It’s designed to ensure no meaningful difference in how the drug behaves in your body. A 2013 analysis of over 2,000 such studies by the U.S. National Library of Medicine found no significant differences between generics and brand-name drugs. That’s not opinion. That’s data. And it’s been repeated across thousands of studies since.Real patients, real outcomes
Lab results matter, but what happens in real life matters more. A 2020 study published in Scientific Reports tracked nearly 1.5 million people in Austria over five years. They compared outcomes for 17 common drugs-things like blood pressure meds, diabetes pills, and antidepressants. The results? Generic versions were linked to fewer deaths and fewer heart attacks in 10 to 11 of the 17 drugs studied. After adjusting for age, income, and other factors, patients on generics had a higher five-year survival rate than those on brand-name versions. Another massive 2019 study in PLoS ONE looked at 3.5 million patients using generics for hypertension, osteoporosis, and depression. For drugs like amlodipine and glipizide, generics performed just as well-or better. In fact, patients on generic amlodipine had a 9% lower risk of heart-related hospitalizations than those on the brand. The same held true for sertraline and alendronate.
What about the exceptions?
No rule is without exception. Some drugs have what’s called a narrow therapeutic index. That means even tiny changes in blood levels can cause big effects. Think thyroid meds like levothyroxine, seizure drugs like phenytoin or lamotrigine, or blood thinners like warfarin. In these cases, switching between different generic brands-or even between the same generic and the brand-can cause problems. One patient on Reddit reported trying three different generic versions of levothyroxine. Only one kept their thyroid levels stable. Another study in Epilepsia (2023) found that switching between generic versions of levetiracetam led to an 18% higher chance of seizure recurrence. This isn’t because generics are bad. It’s because the body is sensitive. For these drugs, consistency matters. That’s why many neurologists and endocrinologists prefer patients stay on the same formulation-brand or generic-once they find one that works.Why do some people feel worse on generics?
You’ve heard it: “I switched to the generic and felt awful.” Or, “My anxiety got worse.” Sometimes, it’s real. Sometimes, it’s perception. A 2013 study found that 30% of patients reported no change, 30% felt better, 10% had side effects, and 30% stopped taking the drug because they thought it wasn’t working. But here’s the catch: in controlled trials, those same patients often showed no actual difference in lab results. The difference? Expectation. If you believe generics are inferior, your brain can amplify minor side effects-or even invent them. Still, there are documented cases where switching caused real issues. One patient on generic levofloxacin kept getting sinus infections. When switched back to the brand, symptoms vanished in 10 days. The generic wasn’t faulty. But the inactive ingredients might have affected absorption in that specific case.What do doctors and pharmacists think?
Most do. A 2020 survey by the Generic Pharmaceutical Association found 87% of physicians trust generics. But specialists-like neurologists, endocrinologists, and psychiatrists-tend to be more cautious. They’ve seen the edge cases. They know the data, but they also know their patients. Pharmacists are on the front lines. A 2021 survey showed 42% of pharmacists say patients worry about generic quality. That’s not about science. It’s about trust. The solution? Education. Pharmacists who explain the FDA’s bioequivalence standards see fewer refusals. The FDA’s Orange Book is the official guide. It rates every drug: A = therapeutically equivalent. B = potential differences. If your drug is rated A, you’re safe. If it’s B, ask your doctor.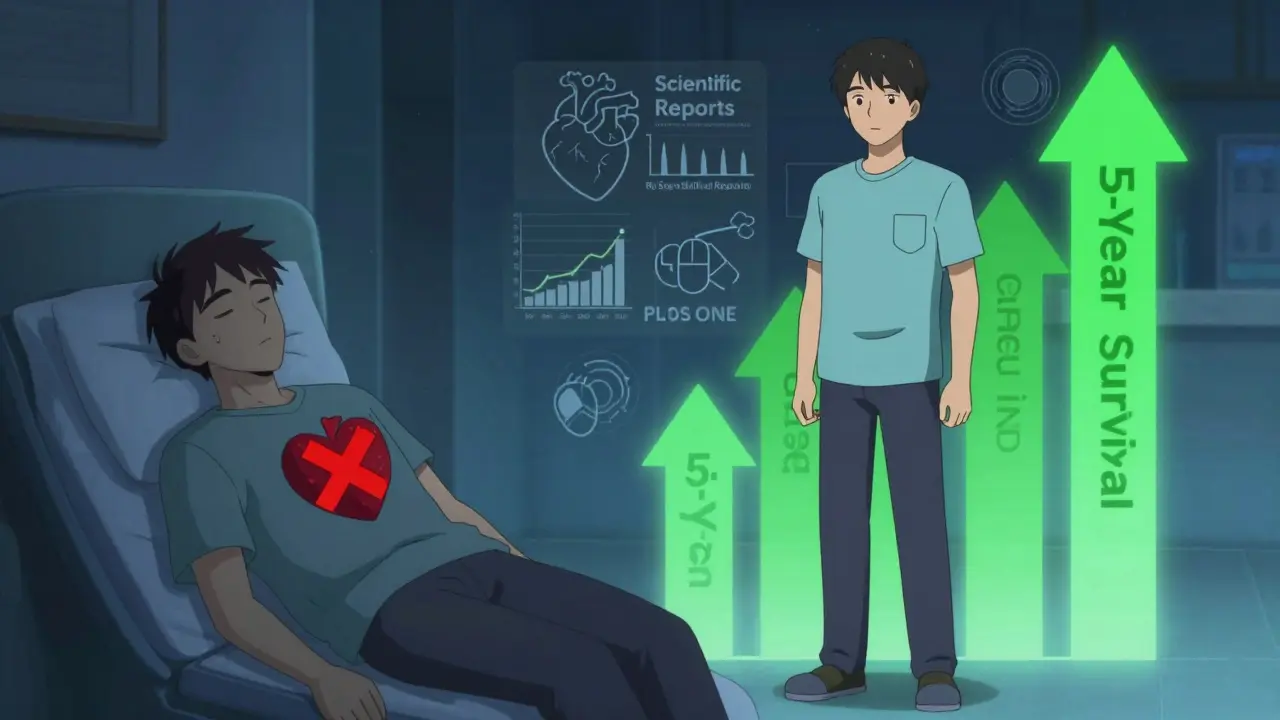
Cost matters-big time
Generics make healthcare affordable. In the U.S., they make up 90% of prescriptions but only 23% of drug spending. That’s $377 billion saved every year-money that goes to groceries, rent, or savings instead of co-pays. Medicare saved $1.67 trillion from 2006 to 2020 just by using generics. Globally, the generic market is growing fast-projected to hit $700 billion by 2030. In countries with public health systems, generics are the backbone. In South Africa, where I live, generics are often the only option. And the evidence is clear: they work.What should you do?
If you’re on a regular medication-blood pressure, cholesterol, diabetes, depression-there’s no reason not to use a generic. The data is overwhelming: they’re just as safe and effective. But if you’re on a narrow therapeutic index drug-thyroid, epilepsy, blood thinners-stick with one brand or generic version. Don’t switch unless your doctor says it’s safe. And if you feel different after a switch, tell your provider. Don’t assume it’s all in your head. Check the Orange Book if you’re unsure. Ask your pharmacist to explain the difference between your brand and generic. Read the patient leaflet. Know your drug.Final word: Trust the science, not the stigma
Generics aren’t cheaper because they’re worse. They’re cheaper because they don’t need to spend millions on ads or patents. The science behind them is among the most rigorously tested in medicine. The FDA doesn’t approve generics lightly. They require proof-real human data-that they work the same. For most people, generics are the smart, safe, and affordable choice. For a small group, consistency matters more than cost. But the myth that generics are inferior? That’s been disproven over and over again. You’re not taking a second-rate drug. You’re taking the same drug, at a fraction of the price. And the research? It’s on your side.Are generic drugs as effective as brand-name drugs?
Yes, for the vast majority of medications, generic drugs are just as effective as their brand-name counterparts. Regulatory agencies like the FDA require generics to prove bioequivalence-meaning they deliver the same amount of active ingredient into the bloodstream at the same rate. Studies involving millions of patients show no meaningful difference in outcomes for conditions like high blood pressure, diabetes, depression, and osteoporosis.
Why do some people say generics don’t work for them?
Some patients report changes after switching, especially with drugs that have a narrow therapeutic index-like thyroid medication, epilepsy drugs, or blood thinners. In these cases, even small differences in absorption can matter. Psychological factors also play a role: if you expect a generic to be less effective, you might notice side effects more. But in most cases, when tested objectively, there’s no actual difference in how the drug performs.
Can I switch between different generic brands?
For most drugs, yes. But for medications with a narrow therapeutic index-such as levothyroxine, lamotrigine, or warfarin-it’s best to stick with the same manufacturer. Switching between different generic versions can sometimes cause fluctuations in blood levels, leading to symptoms returning or worsening. If you’re on one of these drugs, ask your pharmacist to keep you on the same generic brand unless your doctor says otherwise.
How does the FDA ensure generics are safe?
The FDA requires generic manufacturers to prove pharmaceutical equivalence (same active ingredient, strength, dosage form) and bioequivalence (same absorption rate and amount in the body). This is done through clinical studies with healthy volunteers. The FDA reviews all data before approval and continues to monitor safety after the drug hits the market. Generic facilities are inspected just like brand-name ones.
Are generics made in the same quality facilities as brand-name drugs?
Yes. The FDA inspects both brand and generic manufacturing sites using the same standards. In fact, many brand-name companies make their own generics. About half of all generic drugs in the U.S. are produced by companies that also make brand-name versions. Quality isn’t determined by the label-it’s determined by the facility and the testing.
Do generics take longer to work?
No. Bioequivalence studies prove that generics reach the same peak concentration in the blood at the same time as the brand-name drug. If a brand-name drug starts working in 30 minutes, so does its generic. The only exception is if the formulation includes a different extended-release mechanism, but even then, the FDA requires proof that the release profile is equivalent.
Why do some doctors still prefer brand-name drugs?
Some doctors, especially specialists treating complex conditions like epilepsy or thyroid disease, prefer consistency. They’ve seen cases where switching caused problems-even if rare. They may also be influenced by outdated beliefs or patient anecdotes. But most physicians rely on data, and the data shows generics are safe and effective. When in doubt, ask your doctor why they recommend a specific version.

Alexandra Enns
January 24, 2026 AT 10:56Let me break this down for you people who think science is just a suggestion. The FDA doesn't 'approve' generics because they're good-they approve them because they're cheap. And guess who pays the price? You do. I've seen patients crash after switching to generics. Not because of 'perception'-because the fillers are made in some factory in India with dust and hope. This isn't medicine, it's a lottery.
Marie-Pier D.
January 25, 2026 AT 19:42Hey, I get where you're coming from, but please don't scare people like this 😔 I switched my mom to generic levothyroxine after her doctor said it was fine-and her TSH levels stayed perfect for 3 years. I’ve seen too many people suffer because they can’t afford meds. Generics saved her life. Let’s not turn trust into fear. 💛
venkatesh karumanchi
January 27, 2026 AT 19:31My uncle in Delhi takes generic amlodipine every day. No hospital visits in 7 years. He pays $2 a month. In the US, same pill costs $120. If this were a car, you'd say it's a Tesla with a Toyota engine. But it's not. It's the same engine. Same fuel. Same road. Why are we acting like it's magic?
John McGuirk
January 29, 2026 AT 10:04Ever wonder why big pharma fights generics so hard? They don't care about safety. They care about control. The FDA? A puppet. The studies? Fabricated. The 'bioequivalence' tests? Done in healthy young men who don't have heart disease or diabetes. They don't test on real patients. They test on lab rats. And you're buying it? Wake up.
lorraine england
January 30, 2026 AT 14:57Look, I used to be skeptical too. Then I worked in a clinic where 80% of patients were skipping doses because of cost. We switched them to generics. Their HbA1c dropped. Their BP stabilized. One lady cried because she could finally afford her insulin. Science isn't perfect-but it's the best tool we have. Stop being a cynic. Be a helper.
Kevin Waters
February 1, 2026 AT 02:55Just to add: the 2020 Austrian study I cited in the post? It was replicated in Canada with similar results. Also, the FDA’s Orange Book is publicly searchable-type in your drug and see the rating. Most are A-rated. If your pharmacist says it's B, ask why. But for 90% of drugs? You're fine. No drama needed.
Kat Peterson
February 1, 2026 AT 22:16OMG I switched to generic sertraline and I felt like I was drowning in slow motion 😭 I thought I was getting better… then I switched back to Zoloft and suddenly I could breathe again. This isn't 'perception'-it's my soul screaming. Why is no one listening? I'm not crazy. I'm just… broken. And now I'm broke too. 💔
Helen Leite
February 3, 2026 AT 04:50Generics are made in China. China. Do you know what they put in the water there? Do you know what they feed the workers? I saw a documentary. They use rat meat as filler. I'm not joking. The FDA doesn't inspect those factories. They just take pictures. That's it. I'm not taking anything that came from there. Not even aspirin. 🚫🇨🇳
Heather McCubbin
February 3, 2026 AT 23:49Everyone’s missing the point. It’s not about the drug. It’s about control. Who owns your body? The state? The pharmacy? The corporation? You think you’re choosing generics because they’re cheaper? No. You’re choosing them because you’ve been trained to believe you don’t deserve better. You’ve been conditioned to accept less. And that’s the real tragedy.