Future Approaches to Changing Perceptions of Generic Drugs
 Dec, 30 2025
Dec, 30 2025
Most people don’t realize that generic drugs make up 90% of all prescriptions filled in the U.S. - yet they cost just 12% of what brand-name drugs do. That’s not a typo. For every $100 spent on prescriptions, only $12 goes to generics, even though nine out of ten pills taken are generic. So why do so many patients still hesitate? Why do some doctors still whisper, "I’d rather prescribe the brand"? The answer isn’t science. It’s psychology.
Why We Still Doubt What Works
Generic drugs aren’t knockoffs. They’re exact copies - same active ingredient, same dose, same way of working. The FDA requires them to be bioequivalent: meaning they deliver the same amount of medicine into your bloodstream at the same speed as the brand-name version. No wiggle room. No exceptions. Yet, in a 2025 survey, 78% of physicians said their patients still worry generics won’t work as well. Why? Because of how they look. A pill’s color, shape, or imprint triggers subconscious associations. If you’ve taken a blue pill for years labeled "Lipitor," and suddenly your prescription comes as a white oval with "ATV" on it, your brain doesn’t see equivalence. It sees risk. It sees change. It sees something cheaper - and in our minds, cheaper often means worse. This isn’t just about pills. It’s about trust. People don’t distrust generics because they’re bad. They distrust them because they’re unfamiliar. And when your health is on the line, familiarity feels safer - even if it costs ten times more.The New Wave: Biosimilars and Complex Generics
The game is changing. Generic drugs are no longer just aspirin or metformin. In 2025, the FDA approved six new biosimilars for denosumab - drugs used to treat osteoporosis and bone cancer. These aren’t simple pills. They’re complex biologic medicines made from living cells. The original brands, Prolia and Xgeva, cost over $2,000 per injection. The new biosimilars - Bildyos, Aukelso, Enoby - cost 30% less. And they work just as well. But here’s the catch: patients don’t know the difference between a generic pill and a biosimilar injection. To them, it’s all "cheap medicine." And that’s where perception breaks down. Hospitals are seeing real wins. In oncology units, switching to generic injectables has freed up budget to treat more patients - without a single reported drop in effectiveness. But outside the hospital? Most patients still think biosimilars are "experimental." The truth? These drugs go through more testing than brand-name drugs ever did. The FDA requires biosimilars to prove they’re nearly identical in structure, function, and safety. Yet marketing budgets for brands still dwarf those for generics. A single TV ad for a branded GLP-1 drug can cost $50 million. A generic? Nothing. No billboards. No celebrity endorsements. Just a small label on the pharmacy shelf.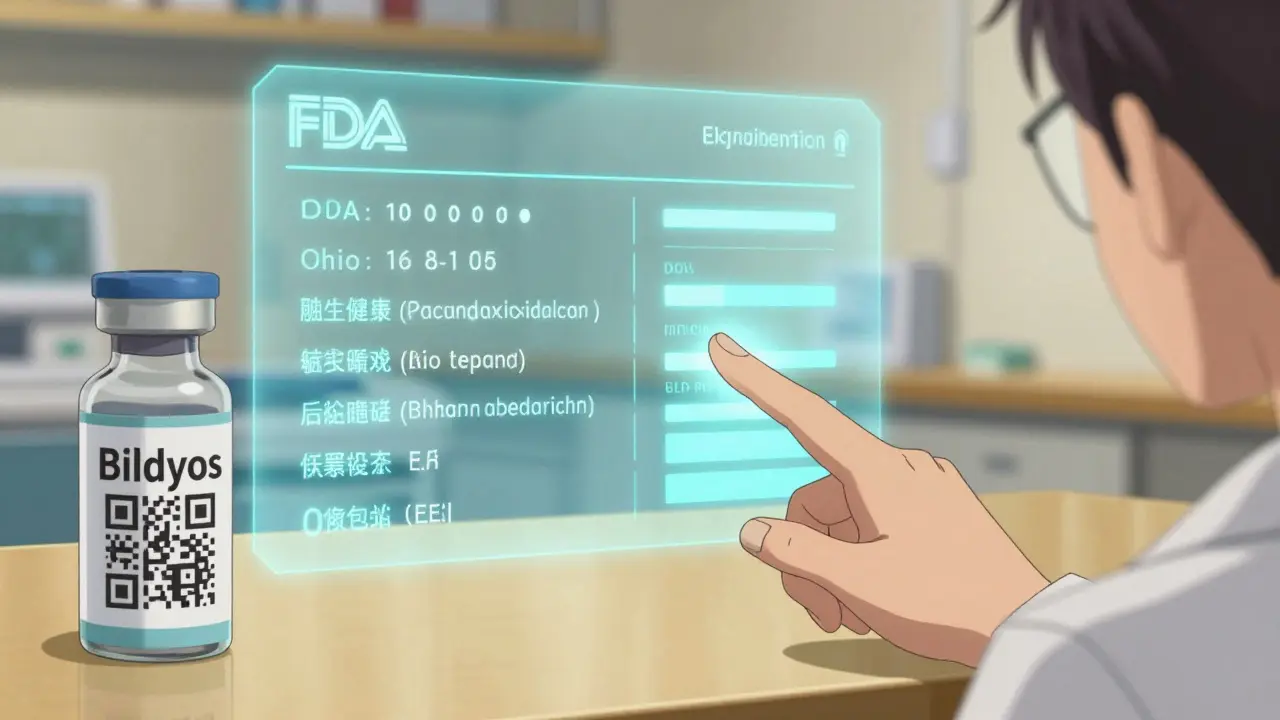
Price Isn’t the Only Problem - Supply Is
In 2025, there were still 270 active drug shortages on the FDA’s list. Many of them were generics. Why? Because manufacturing small, low-margin pills isn’t profitable for big pharma. So companies leave the market. When a generic runs out, patients panic. They switch back to the brand - even if it costs $300 a month instead of $10. That’s where CivicaScript comes in. Founded by hospital pharmacists, Civica doesn’t just make generics. It guarantees supply. They partner directly with hospitals, produce drugs in U.S.-based facilities, and sell them at transparent, fixed prices. No middlemen. No bidding wars. No stockouts. Their model works because it removes the fear of running out. And when patients know their medication will always be there - not just cheap, but reliable - trust grows. Domestic production is now a key strategy. The U.S. used to rely on overseas factories for 80% of its generic drugs. Now, federal incentives are pushing manufacturing back home. More factories. Fewer shortages. More predictable supply. That’s not just logistics - it’s perception change in action.Education That Actually Works
Telling patients "generics are just as good" doesn’t work. They’ve heard it before. What does work? Showing them. In a 2025 pilot program by the American Medical Association, doctors sat down with patients on chronic medications - hypertension, diabetes, thyroid - and showed them side-by-side lab results. Same blood pressure. Same HbA1c levels. Same cholesterol. One group took the brand. The other took the generic. After six months, the numbers were identical. The result? A 35% drop in patient concerns about generics. It wasn’t about cost. It wasn’t about science jargon. It was about proof they could see. Real data. Real outcomes. Their own bodies. Pharmacists are now doing the same thing. Instead of just handing over a new bottle, they say: "This is the same medicine your doctor prescribed. It’s just made by a different company. Here’s the report from the FDA showing how it matches. Would you like to see your last lab results compared?" Simple. Direct. Human.
Technology Is Building Trust Behind the Scenes
You can’t see it, but technology is quietly reshaping how generics are trusted. Blockchain is being used to track every batch of generic medicine from factory to pharmacy. AI analyzes supply chains to predict shortages before they happen. Digital tools let patients scan a pill’s imprint and instantly see its FDA approval status, manufacturing location, and bioequivalence data. Imagine this: You pick up your generic blood thinner. You open your pharmacy app. You scan the label. A pop-up shows: "This batch was made in Ohio, tested by FDA, bioequivalent to Xarelto. 97% of patients report same effectiveness. No recalls in 2025." That’s not marketing. That’s transparency. And transparency kills suspicion. In 2025, companies like Civica and others began embedding QR codes on generic packaging. Not for discounts. For facts. And patient engagement jumped 40% in clinics that used them.What’s Next? The Shift from Cost to Confidence
The future of generic drugs isn’t about being cheaper. It’s about being trusted. We’re past the point where price alone drives adoption. The market is maturing. Generic prices are stabilizing. The real opportunity now is in specialty generics and biosimilars - drugs that treat cancer, autoimmune diseases, and rare conditions. These are expensive to make. But they’re also life-changing. The question isn’t whether generics can compete. They already have. The question is: Can we finally stop treating them like second-class medicine? The answer lies in three things:- Consistent supply - no more shortages that scare patients into paying more.
- Transparent proof - letting people see the data, not just hear promises.
- Human connection - pharmacists and doctors who take the time to explain, not just dispense.
Are generic drugs really as effective as brand-name drugs?
Yes. By law, generic drugs must contain the same active ingredient, strength, dosage form, and route of administration as the brand-name version. The FDA requires them to be bioequivalent - meaning they work the same way in the body. Studies show no meaningful difference in effectiveness or safety between generics and brand-name drugs for the vast majority of medications.
Why do some patients switch back to brand-name drugs?
Most switch because of perception, not science. A change in pill color, size, or brand name can trigger anxiety, even if the medicine is identical. Insurance changes often force switches, and when patients feel uncertain, they revert to what they recognize - even if it costs more. Forty-two percent of patients who switch back cite perceived quality differences, despite no clinical evidence supporting that belief.
What’s the difference between a generic drug and a biosimilar?
Generics are exact copies of small-molecule drugs, like pills for blood pressure or diabetes. Biosimilars are highly similar versions of complex biologic drugs - made from living cells, like those used for cancer or autoimmune diseases. Biosimilars aren’t identical (because biological molecules are too complex), but they must prove near-identical function and safety. They offer 15-30% cost savings, compared to 80-85% for traditional generics.
Why are generic drug shortages still happening in 2025?
Many generic drugs are low-margin products, so manufacturers sometimes exit the market if profits drop. Overseas production delays and raw material shortages also play a role. In 2025, there were still 270 active shortages on the FDA list, mostly for older, low-cost generics. Solutions include domestic manufacturing, like CivicaScript’s model, and federal incentives to keep production stable.
Can technology help improve trust in generic drugs?
Yes. Blockchain tracks drug batches from factory to pharmacy, ensuring authenticity. AI predicts supply chain issues before they cause shortages. QR codes on packaging let patients scan and instantly see FDA approval data, manufacturing location, and bioequivalence reports. In clinics using these tools, patient confidence in generics rose by 40% in under a year.
How are hospitals changing perceptions of generics?
Hospitals are leading the way by using generics - especially injectables and biosimilars - in high-stakes settings like oncology. When patients see that their cancer treatment works just as well on a generic version, and that the hospital saved money to treat more people, trust builds naturally. Pharmacists are also educating patients directly, showing lab results side-by-side to prove equivalence. These hands-on approaches have reduced patient concerns by up to 35% in pilot programs.

Paul Huppert
December 31, 2025 AT 14:10Had my blood pressure med switched to generic last year. Thought I’d feel weird or something. Didn’t. Same results. My BP’s been stable as hell. Honestly? I’m just glad I’m not paying $300 a month for the same damn pill.
Brady K.
January 2, 2026 AT 01:27Let me get this straight-we’ve got a pharmaceutical-industrial complex that spends $50M on a TV ad to convince you your body can’t tell the difference between a blue pill and a white one… but somehow, the FDA’s bioequivalence standards are the ‘suspicious’ part? 😂
It’s not that generics don’t work. It’s that the brand-name companies paid for your fear. They sold you anxiety as a brand extension. And now we’re all just paying the subscription fee for delusion.
Meanwhile, the real innovation? Hospitals using QR codes to show patients their own lab data. That’s not marketing. That’s accountability. The system’s broken. But the solution? It’s already in the pharmacy.
Kayla Kliphardt
January 3, 2026 AT 12:28I’ve been on generics for years. Never had an issue. But I get why people panic. My grandma switched to a new generic for her thyroid med and swore it ‘felt different.’ Turned out her pharmacy gave her a different manufacturer. Same ingredients. Different color. She cried. I had to sit with her for an hour just to calm her down.
It’s not about science. It’s about the ritual. The pill is part of the identity of being sick. Change that, and you shake the whole story.
Urvi Patel
January 4, 2026 AT 15:21Who even cares about generics anymore I mean look at the world we live in AI is curing cancer and we still arguing about pill colors lmao
anggit marga
January 5, 2026 AT 16:16USA always acting like generics are some kind of conspiracy when other countries have been using them for decades and living longer I mean why do you think Europe has better life expectancy dumbass
Joy Nickles
January 6, 2026 AT 00:58Okay so… I’m just gonna say it… I think this whole thing is a scam… I mean, I took a generic version of my antidepressant… and I swear… I felt… *different*… like… emotionally… flat… like… my soul was… muted…? I didn’t want to cry… or laugh… or even scroll TikTok… and I’m like… is this… is this the FDA…? Did they… just… *dilute*… my emotions…? 😭
And then I went back to brand… and boom… tears… joy… existential dread… all back… so… maybe… maybe… it’s not just the pill… maybe… it’s the… *brand*… of my trauma…?
Marilyn Ferrera
January 6, 2026 AT 15:10Real talk: if you’re still skeptical about generics, you’re not being cautious-you’re being manipulated. The FDA doesn’t approve generics based on trust. They approve them based on blood levels, pharmacokinetics, and clinical outcomes. If your doctor says, ‘I’d rather prescribe the brand,’ ask them: ‘Why? Is it because it works better-or because they paid for your lunch?’
Generics aren’t a compromise. They’re the default. The brand is the luxury add-on.
Brandon Boyd
January 7, 2026 AT 03:13Man, I used to think generics were sketchy too-until my kid needed insulin. Brand cost $500 a vial. Generic? $40. Same exact thing. We switched. No side effects. No drama. Just more money for groceries and soccer camp.
Stop letting Big Pharma tell you what to fear. Your body knows the difference. And if it’s not screaming, it’s probably fine.
Trust your science. Not your ad.
Frank SSS
January 8, 2026 AT 20:37So let me get this straight-you’re telling me that a $2,000 injection and a $1,400 injection are basically the same… but we’re still acting like the cheaper one is some kind of medical experiment? 🤡
Meanwhile, my cousin got a biosimilar for her rheumatoid arthritis and saved $800 a month. She’s now taking her mom to Hawaii. The brand? Still paying for her therapist.
It’s not about the drug. It’s about who’s profiting from your confusion.
Harriet Hollingsworth
January 10, 2026 AT 08:00THIS IS WHY AMERICA IS FALLING APART. People are too lazy to read the FDA label. Too scared to try something new. Too addicted to branding. You’d rather pay $300 for a pill than learn that the white oval with ‘ATV’ on it is the same medicine your grandfather took. Pathetic.
Bennett Ryynanen
January 12, 2026 AT 02:33My dad’s on a generic for his heart med. He used to freak out every time the pill changed shape. I showed him the FDA bioequivalence report. He didn’t believe it. So I took him to his pharmacist. The guy pulled up his last three lab results-side by side. Same numbers. Same everything.
He looked at me and said, ‘So… I’ve been paying extra for a blue pill this whole time?’
Yeah, dad. You have.
Now he asks for generics first. And he’s saving $200 a month. That’s a damn vacation.
Chandreson Chandreas
January 12, 2026 AT 09:36Bro… I live in India… generics are EVERYWHERE… and we don’t have a crisis… we have a system that works 🤝💊
USA you’re overthinking pills like they’re crypto tokens 😅
Same medicine. Same effect. Less money. Win-win. Why is this hard?